Now that we know all matter is made of atoms, we need to understand what an atom is, and how many there are! Keep reading to find out all about ATOMS and the substances they compose called ELEMENTS.
What are ELEMENTS?
- Substances composed of the same atom
- 90 basic substances can be found on Earth
- 28 more elements can be created in laboratories
- Elements can be combined to form other substances

What is an ATOM?
- Smallest whole piece of matter
- 90 different atoms exist on earth
- 28 more atoms are created synthetically in particle accelerators
- Atoms link together to form other particles like Legos

Where do we find information about Atoms and Elements?
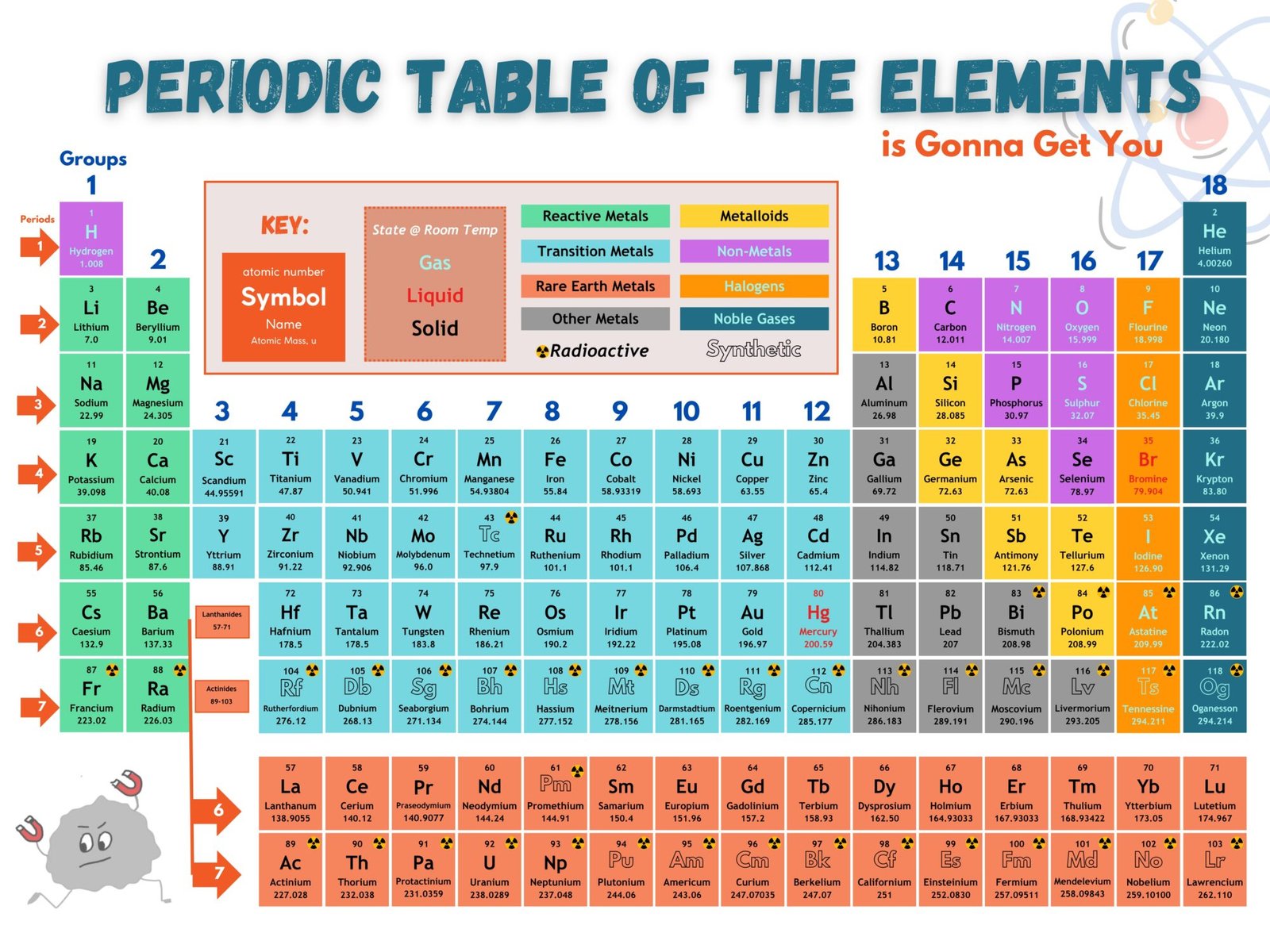
How do we make sense of the Periodic Table boxes?
Atomic Symbols
- Universal shorthand for each element
- First letter always CAPITALIZED, if there’s a second it’s lowercase
- Carbon (C)
- Cobalt (Co)
- Ancient Element symbols often reflect Greek or Latin Names
- Gold (Au) for Aurum (Latin)
- Iron (Fe) for Ferrum (Latin)
- Mercury (Hg) for Hydragyrum (Greek)
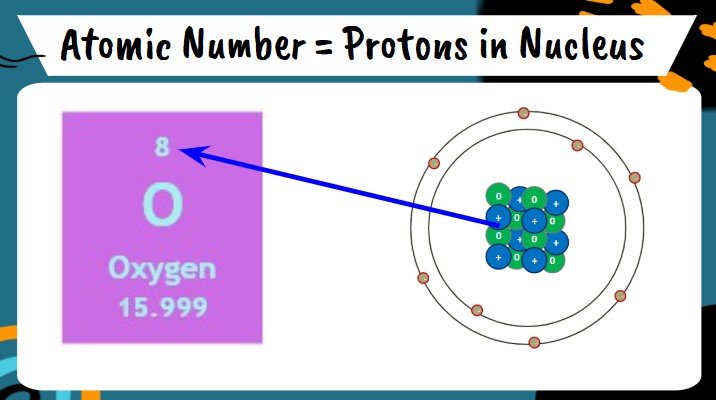
Atomic Number
- Periodic Table is organized by this unique identifier for each atom
- Numbers increase from left to right, then continue in next row
- Represents the number of protons (positively charged smaller particles that make atoms) in the nucleus
- Oxygen has 8 protons
- Carbon has 6 protons
- Gold has 79 protons
Atomic Mass
- Larger number in the box representing the total mass of an atom
- May appear as a decimal because it is an average of all the different versions of atoms (Isotopes) that exist on Earth
- Oxygen has a mass of 16 particles in the nucleus
- Carbon has a mass of 14 particles in the nucleus
- Gold has a mass of 197 particles in the nucleus
- May appear as a decimal because it is an average of all the different versions of atoms (Isotopes) that exist on Earth
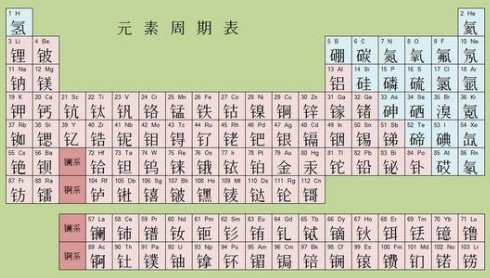
Periodic Tables vary widely depending on Publisher and Language
- Publishers each put their own spin on coloring and fonts
- Advanced chemistry uses tables with extra information about atomic structure
- Atomic names appear in the language of each country
Who created the Periodic Table?
- Russian Chemist Dmitri Mendeleev in 1869
- Instead of just alphabetical lists or lists by atomic mass, Mendeleev’s revolutionary table placed the elements in groups and rows based on repeating patterns in behavior and characteristics known as properties.
- With gaps on his table, he could predict what yet to be discovered elements would be like
What are “synthetic” elements?
- Only 90 elements exist on Earth, but the Periodic Table has 118 named boxes
- Scientists “create” these elements in Particle accelerators by slamming smaller atoms together to create larger ones
- ex. Bismuth (83) + Chromium (24) = Bohrium (107)
- Unfortunately they quickly fall apart so every element larger than Americium (95) is basically useless
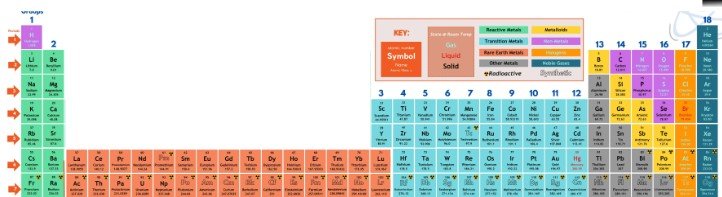
What’s the deal with the 2 rows at the bottom?
The truth is that the periodic table only has 7 rows with the Rare Earth Elements actually belonging in the center of the table causing it to look pretty silly. So the center pieces are typically cut out and placed at the bottom:)

Why is it called the Periodic Table of Elements instead of the Periodic Table of Atoms?
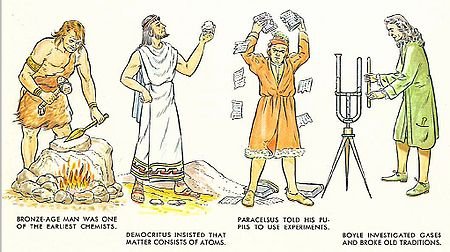
- Early chemists lacked powerful microscopes and particle accelerators, so they studied and discussed elemental substances like Gold and Silver.
- After being hypothesized by Democritus in 400BCE, Atoms weren’t formally discovered until 1808 by John Dalton with the first photograph taken in 2008!
Let’s keep learning…
So if water, soil and air aren’t ELEMENTS on the Periodic Table, but they ARE made of Atoms, what are they?