If there are only 90 types of atoms found on Earth, how do we have over 100 million different substances? Because just as Legos link together to make structures and letters link together to make words, atoms link together to form different substances called COMPOUNDS! Let’s learn:
What is a COMPOUND?
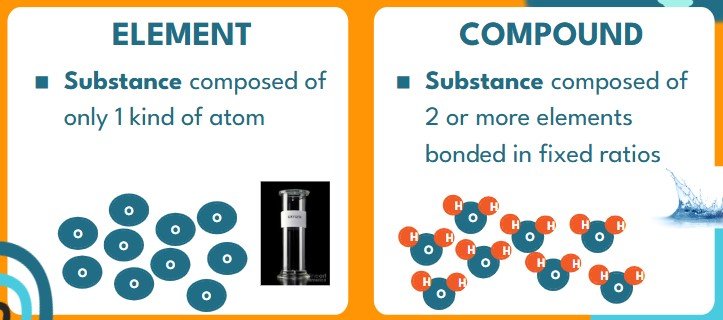
- COMPOUNDS are substances composed of the same molecules formed by combining elements
- Ex: If we take 20g of Hydrogen and 10g of Oxygens we get 30g of Water because the atoms in those substances bond at at 2:1 ratio to make Water (H2O) molecules
- Compounds can be broken apart into the elemental substances they are made from.
What is a MOLCULE?
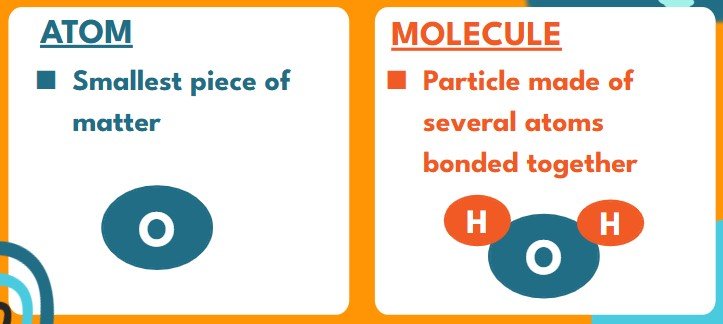
- MOLECULES are the particles created when atoms bond to each other.
- Ex: When two Hydrogens (H) and one Oxygen (O) bond we get an H2O molecule
- Each atom retains it atomic identity, they are just stuck together and move in a unit
- Molecules can be broken apart into the atoms that formed them.
What is a CHEMICAL FORMULA?
- Chemical formulas use Atomic Symbols and subscript numbers to describe how many of which atoms link together to form molecules and therefore create compounds
- Water H2O = 2 Hydrogens + 1 Oxygen
- Methane CH4 = 1 Carbon + 4 Hydrogens


So how do compounds compare to the elements they are made from?
- Just as teenagers act differently when holding hands with their beloved, atoms look and act differently when they bond to each other too!
- When atoms bond, they create compounds that have completely different properties than the elements that compose them.
- Look how different the elements are from the compounds they create below:



Let’s keep Learning…
While compounds account for millions of the substances on Earth, there are still millions more. Keep reading to learn more!


