Just as the ingredients in your pantry combine to form different substances, the 90 atoms on Earth form almost infinite combinations to create all of the substances we come into contact with every day!
PURE MATTER contains only one particle
If someone gave you a “pure gold” necklace, you would expect Gold to be the only thing in that item. Likewise, when you ask someone to pass you the salt at the table, you expect the only thing to come out of the shaker to be salt crystals. Just as our use of the word “pure” infers that we want whatever we are asking for to only be composed of that thing, scientists define PURE MATTER as a substance only composed of one particle!
ELEMENTS ARE PURE!
Elements (Gold included) are considered pure because they are made of only one kind of atom.
- Oxygen (O) is made of Oxygen atoms
- Silver (Ag) is made of Silver atoms
- Sodium (Na) is an element made of Sodium atoms
- Gold (Au) is made of Gold atoms –>

COMPOUNDS ARE PURE!
Even though compounds are made of multiple elements combined, the elements are combined in fixed ratios to form consistent molecules. Because every molecule in a compound is identical, they are also considered pure.
- Carbon Dioxide is made of CO2 molecules
- Table salt is made of NaCl molecules
- Water is made of H2O molecules –>

MIXTURES AREN’T PURE
When a substance is made of different particles, we consider it a mixture. When we bake, each ingredient has unique flavor and texture properties because it is made of different molecules. So when combine ingredients, we’re creating a mixture with several different molecules!




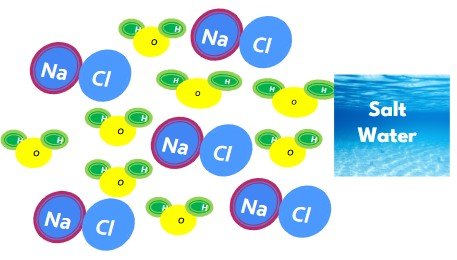
The mixture of salt water is made of pure compounds table salt (NaCl) and water (H2O):
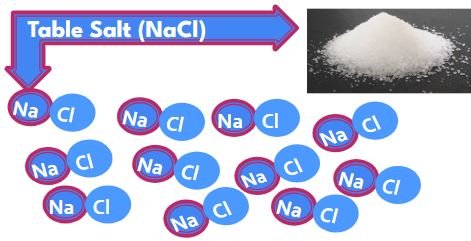
TABLE SALT (pure)
- Compound NaCal
- Pure substance made of only NaCl molecules
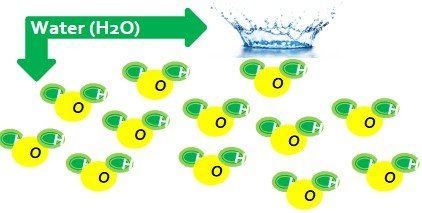
WATER (pure)
- Compound H2O
- Pure substance made of only H2O molecules
SALT WATER (mixture)
- Mixture of two compounds
- Contains 2 separate particles H2O and NaCl
So many ways to mix
Mixture of Elements
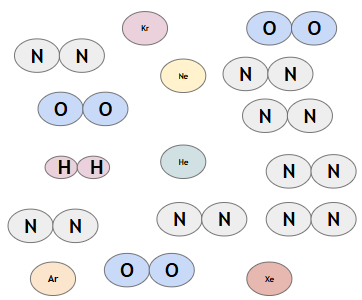
Mixture of Compounds
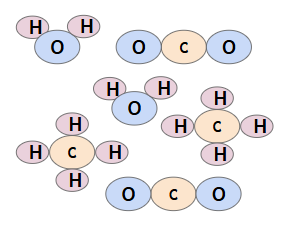
Mixture of Elements & Compounds

Mixtures are further classified based on how well they are mixed:
HOMOGENEOUS MIXTURES
- All particles evenly mixed
- Cannot see any ingredient chunks separate from the well blended mixture that looks all the same throughout
- Prefix “homo-” means same
- Ex. Fruit Smoothie


HETEROGENEOUS MIXTURES
- Particles are not evenly mixed
- Can see at least one the ingredients standing out from the rest of the mixture
- Prefix “hetero-” means different
- Ex. Fruit Salad


Can you categorize these mixtures? (scroll to very bottom for answers)
Need a little more explanation?
Ready to keep learning?

Answers for mixture categorization
- Homogenous: Chocolate Pudding, Obsidian, Hot Sauce, Frosty, Peanut Butter Cookie
- Heterogeneous: Caramel Macchiato, Granite, Chocolate Chip Cookie, Salsa, Chili, Peanut Butter Cup, Hot Fudge Sundae













